The Breast and Ovarian Surveillance Service (BOSS) Cohort Study is an ongoing prospective study consisting of women and men with a familial risk for breast and/or ovarian cancer, recruited in 2005–2013 from the cancer genetics clinic at the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center (Baltimore, MD).
The Breast and Ovarian Surveillance Service (BOSS) Cohort Study is an ongoing prospective study consisting of women and men with a familial risk for breast and/or ovarian cancer, recruited in 2005–2013 from the cancer genetics clinic at the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center (Baltimore, MD).
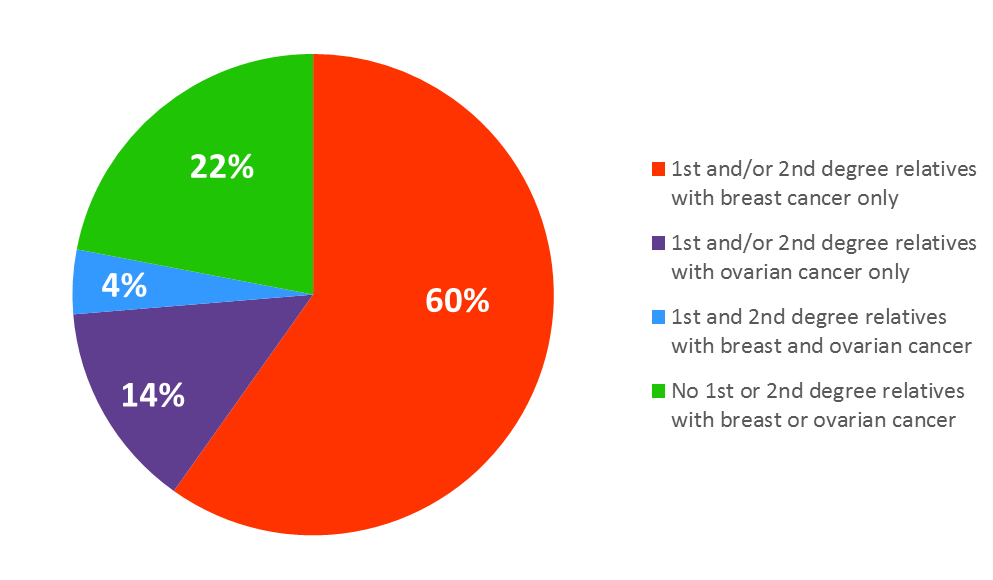
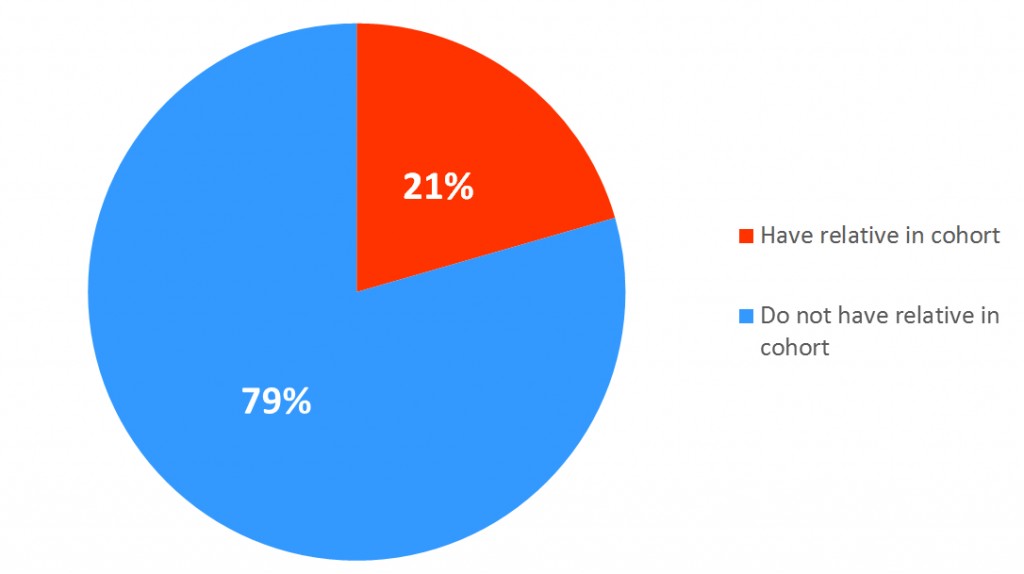
At enrollment, after informed consent was obtained, participants were asked to complete an extensive baseline questionnaire on demographic characteristics, weight, height, lifestyle factors, including physical activity, and breast cancer risk factors, cancer history and treatments, and medication use. A detailed family history and plasma, serum, and DNA samples were obtained at this time.
Follow-up questionnaires are administered every 3 years and have been completed by participants two times since enrollment with >90% compliance. All cancer diagnoses were based on pathology records and all treatment information was confirmed by medical records.
Who is eligible to join the BOSS cohort?
- Individuals 18 years of age or older;
- Women and men with a family history of breast cancer in one first or second-degree relative(s) on the same side of the family or;
- Women and men with a family history ovarian cancer in one first or second degree relative or;
- Women and men with a known breast cancer gene mutation (BRCA 1/2) or;
- Women and men without a family history of breast cancer who were diagnosed with breast cancer at 40 years or age or younger or;
- Women without a family history of ovarian cancer diagnosed with ovarian cancer at any age
What does participation involve?
- Completing a detailed health questionnaire
- Donating blood
- Reviewing and signing a consent form & medical record release forms
- Allowing the study to borrow mammogram(s) to assess breast density
- Updating health, cancer family history and contact information every 3-4 years
- Willing to be informed about new sub-studies to take part in if eligible
Follow-Up
Thank you for your ongoing support of the Johns Hopkins Breast and Ovarian Cancer High-Risk Cohort. Your willingness to keep your health information updated with us is critical and allows us to continue our research efforts to learn about factors associated with both breast and ovarian cancer. We hope that results of our research will benefit others in the future. Furthermore, your updated information supports our thinking about research ideas and in requesting funding to explore new studies and interventions.
Description:
Every 3 years after joining the cohort, you will be contacted for a brief follow-up survey, asking questions such as current contact information, personal health conditions, cancer family history changes, medication use, risk factors and life style factors. All participants are made aware of this at time of enrollment. Follow-up surveys can be completed via a secure website, by a telephone interview with a staff member (takes about 15 minutes), or by a mailed paper version.
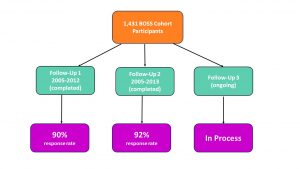
Follow Up Response Rates:
We are very pleased to share that our average follow up response rate is approximately 90%. Many participants have completed two cycles of updates and a third cycle is ongoing. We are delighted to keep in touch and also hear any comments.
Keep Us Updated:
If you are unsure if you have completed a recent follow-up or have new information to share with us, please call the Cohort Study Line at (443) 287-6144.
Cohort Statistics
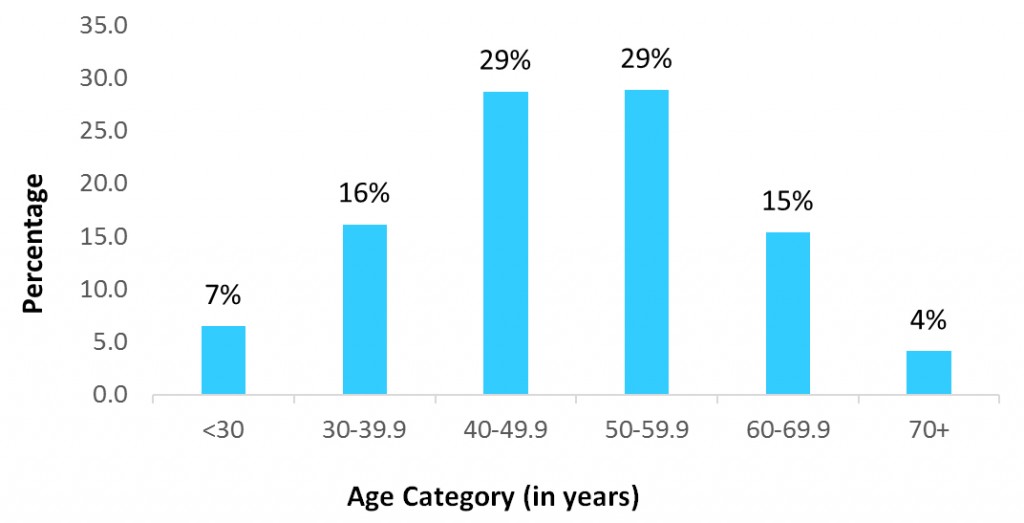
To date, the Breast and Ovarian Surveillance Service (BOSS) Cohort Study has enrolled 1,432 participants, of which 1,409 are females and 23 are males. Participants provide updates every three to four years regarding their demographic characteristics, personal health, medication use, family history of cancer, lifestyle factors, and breast cancer risk factors. We are pleased to share a few summary statistics about cohort participants that you may find interesting.
Current Location of Study Participants
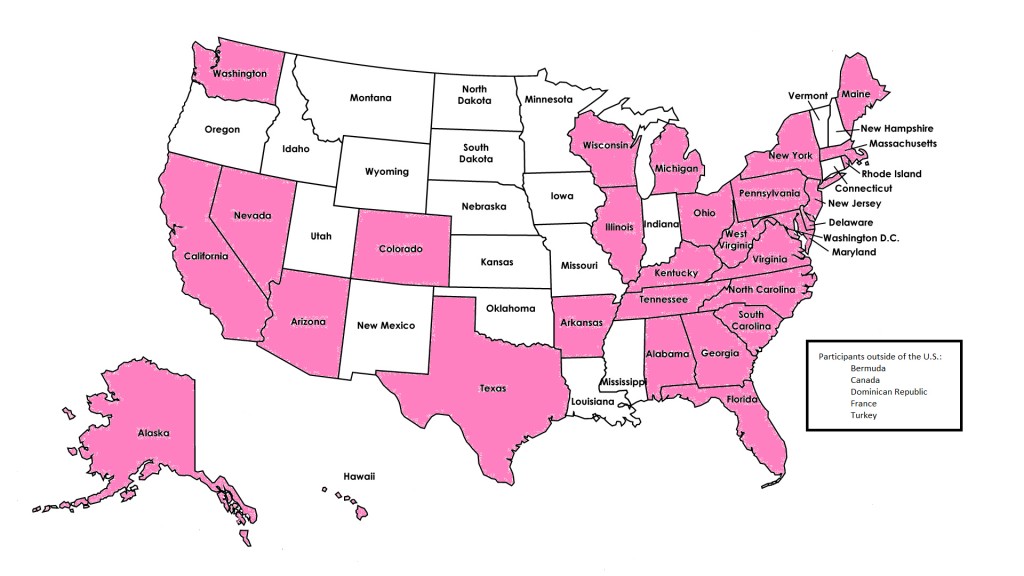 Individuals were primarily recruited to the cohort from the cancer genetics clinic at the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center (Baltimore, Maryland). Although the majority of enrollees reside in the Mid-Atlantic region, we have representation from more than 30 states and five countries other than the US.
Individuals were primarily recruited to the cohort from the cancer genetics clinic at the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center (Baltimore, Maryland). Although the majority of enrollees reside in the Mid-Atlantic region, we have representation from more than 30 states and five countries other than the US.
 Research Biospecimens Collected
Research Biospecimens Collected
The BOSS cohort specimen repository contains:
- Over 1,770 blood samples
- Over 300 urine samples
- Over 120 breast fluid/aspirate samples
- Over 140 breast fine needle aspirate samples
- Over 60 fresh frozen breast tissue samples
- Over 12,700 tissue/slides from breast and ovarian procedures and/or surgeries
All biospecimens were obtained with permission from participants.
 Pathology Reports and Records Collected
Pathology Reports and Records Collected
The BOSS cohort data repository contains:
- Over 2,000 pathology reports (including breast biopsies, lumpectomies, mastectomies, oophorectomies and hysterectomies, as well as other types of cancer surgeries) collected and abstracted
- Over 12,700 tissue slides (including breast cancer, ovarian cancer, benign breast disease and oophorectomy)
- Over 870 genetic testing results
- Cancer Treatment information from medical records (ongoing)
- Mammography records (ongoing)
- Death certificates
- Questionnaire data from enrollment and ongoing follow-ups.
All records were obtained with permission from participants.